Commitment
We are committed to making everything we do count towards the best therapies and supportive care for cancer patients—from the products we license to the rigorous standards we apply as a pharmaceutical manufacturer. At Helsinn we are committed to extending the reach of our quality care and use our expertise to fulfill more unmet needs and build better todays for more patients worldwide.
Our Compliance Program
To conduct its business with ethics and integrity, Helsinn has established and maintains an effective Compliance Program. This program has been developed in accordance with applicable laws, regulations, Helsinn Policies and industry codes.
Helsinn’s Compliance Program includes:
- Compliance Officers and Business Ethics Board (”BEB”) charged with the responsibility for operating and monitoring the Compliance Program
- Code of Conduct, Policies and Procedures detailing the Company’s commitment to compliance and setting forth the ethical and compliance principles applicable to all employees
- Regular education and training programs for applicable employees
- Easy access to the Compliance Officers by all employees.
- A reporting process to collect concerns and ask questions
- Policies and procedures to protect the anonymity of employees who raise concerns (as applicable) and to prohibit any form of retaliation against them
- Use of audits and routine monitoring activities to monitor compliance and identify and address risks
- Enforcement of compliance obligations through policies that include corrective measures or penalties for non-compliant behaviors
- Mechanisms to promptly and properly investigate and respond to reports of non-compliance, including processes to initiate corrective measures and to report offenses to the relevant authorities where appropriate
The creation of appropriate standards, policies and procedures, and guidelines establishes a framework of behavior for the company to follow. These standards are based on organizational risks and laws, codes and policies of the countries in which the business activity takes place.
A high level management committee often works with the GCO on operational issues and interfaces with the Board on oversight matters. As such, appropriate authority has been given to the GCO and BEB to make decisions and oversee the compliance program.
Initial and ongoing training for employees is essential to a compliance program to help ensure that they know how to be compliant and to set Helsinn’s expectations of them. Ongoing education and communications are necessary to reinforce training topics and inform relevant audiences about other compliance issues and information.
Ongoing monitoring and auditing can help identify areas of risk where further attention is needed such as policy development or corrective action. Monitoring and auditing can also spot trends that may indicate a need for stronger controls such as further education and training.
A helpline is available for employees to report incidents or concerns and serves as a venue to ask questions. All allegations are promptly investigated.
Go to helsinn.ethicspoint.com
Fair, equitable and consistent methods to manage employees involved in compliance incidents serve as an important part of an effective compliance program and also illustrate the company’s commitment to compliance and ethics. Coordination among the Legal, Human Resources and the Compliance Office can help ensure the proper disciplinary steps are taken based on the specifics of the incident.
If misconduct or a gap in the program has been discovered, appropriate action on behalf of the company should follow. This step shows the company’s resolve to correct the issue once the company becomes aware of it and that the company does not continue to let issues arise in the future. It is very important, once the company is informed of an issue, to address it promptly. This may often include consultation among the Legal, Human Resources and the Compliance Department.
Helsinn Therapeutics (U.S.) Inc. has established a comprehensive Compliance Program in accordance with the April 2003 “Compliance Program Guidance for Pharmaceutical Manufacturers” (“OIG Guidance”) and the “Pharmaceutical Research and Manufacturers of America (PhRMA) Code.” To learn more about Helsinn Therapeutics (U.S.) Inc. Compliance Program or to access our annual declaration of compliance with the California Health & Safety Code please click on the following links:
Helsinn Therapeutics (US)., Comprehensive-Compliance Program
Helsinn Therapeutics (US) Inc., Information for Vermont Prescribers
Anti Corruption Policy
Helsinn firmly rejects all forms of corrupt practices. Corruption has devastating effects on society, on the environment and is a major obstacle to economic development. Helsinn complies with international anti-corruption standards and all applicable laws, regulations, and codes against corruption, such as Article 102 of the Swiss Criminal Code, the U.S. Foreign Corrupt Practices Act, the UK Bribery Act and the Irish Prevention of Corruption (Amendment) Act 2010. Non-compliance with these standards can have severe consequences for Helsinn and the employees concerned.
- We interact with all our stakeholders with the highest level of integrity based on the merits and the science behind our medicines.
- We do not offer or give, directly or indirectly through third parties, anything of value to any person or organization, whether public officials or not, to obtain or retain any undue advantage, including healthcare professionals, government agencies, government officials, employees, health plans, payers, patient organizations and patients.
- We do not offer stakeholders any gifts, sponsorships, grants, donations, hospitality, entertainment or anything of value in return for use, referrals, favorable formulary, or treatment guideline positions, or to obtain any other preferential treatment for Helsinn or its products.
- We do not contract with stakeholders for speaking services, advisory boards, scientific research or any other service in return for use, referrals, favorable formulary, or treatment guideline positions, or any other preferential treatment for Helsinn or its products.
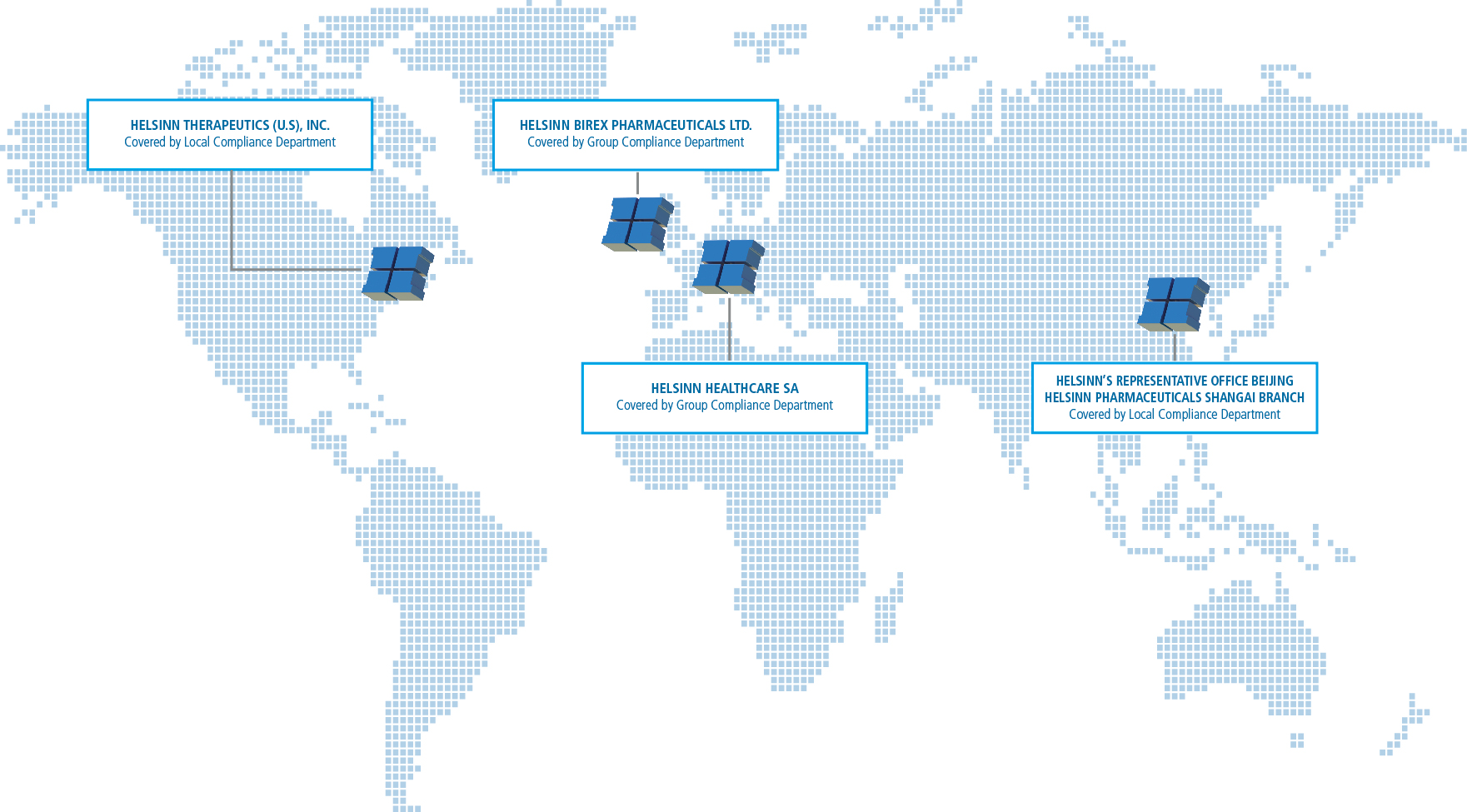
Compliance Team
The Compliance program – led by the Group Compliance Officer based in Helsinn Headquarter in Lugano (CH) – is supported by compliance resources at headquarters and around the world.
Full dedicated teams consisting of Regional Compliance Officers covering all countries in which we are based are a fundamental part of our Compliance team.