Skip to content
![]()
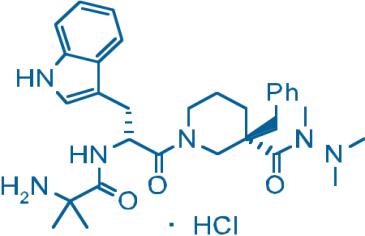
Anamorelin
Anamorelin is a novel, orally available, selective ghrelin receptor agonist that mimics the appetite-enhancing and anabolic effects of the ghrelin hormone.
Development status:
- Approved in Japan
- Phase III global clinical studies (SCALA) completed
